- Introduction
- The use of psychotropic agents is
widespread, as is evident from the fact that
many patients have been treated with them since
their introduction. Concepts of the relationship
between the actions of psychotropic drugs and
the functions of specific brain systems have
particularly progressed through the relatively
brief history of psychopharmacology.
Furthermore, efforts to provide explanations for
drug-induced neurological changes will continue
to focus on synaptic transmitters and their
mechanisms. New approaches in drug development
have been advanced by studies which have
permitted the identification of receptor
subtypes, undetected by traditional
pharmacological approaches, with receptor
heterogeneity providing an opportunity for
greater pharmacological selectivity. All of
these factors must be kept in mind when
attempting to develop comprehensive explanations
of the effects of drugs.
-
- The association between specific clinical
syndromes and predictable responses to
psychotropic drugs has supported the impressive
recent progress in this area. Testable
hypotheses about possible biological bases of
severe psychiatric illnesses have been
stimulated by knowledge of the mechanisrns of
action of psychotropic agents. In recent years,
emphasis has been focused on biogenic amines and
their receptors in the brain, their probable
mediation of many effects of psychotropic drugs
and their possible causal involvement in mental
illness. Antipsychotic or neuroleptic drugs,
which have been used to treat psychoses, have
beneficial effects on mood and thought and
antagonize the neurotransmitter actions of
dopamine in the forebrain. It has, therefore,
been proposed that there may be a state of
functional overactivity of dopamine in the
limbic system or cerebral cortex in
schizophrenia or mania. However, these drugs
carry the risk of producing characteristic side
effects that mimic neurological diseases.
Although the antipsychotic drugs have had a
revolutionary, beneficial impact on medical and
psychiatric practice, much attention has been
given to the disadvantages of treatment with
psychotherapeutic drugs, especially to their
limited efficacy in severe or chronic mental
illnesses, their frequent association with
extrapyramidal neurological effects and their
risk of occasional serious toxic effects.
-
- Antipsychotic drugs which block postsynaptic
dopamine receptors cause extrapyramidal symptoms
that resemble parkinsonism, especially in older
patients. In addition, there is a marked
deficiency in dopaminergic innervation of the
basal ganglia due to degeneration of neurons in
the substantia nigra. The loss of this
catecholamine from the basal ganglia has been
shown to underlie all of the major motor
manifestations of parkinsonism. Restoration of
dopaminergic transmission restores motor
function in parkinsonism and forms the central
strategy in virtually all current drugs regimens
for treatment of the disease. Many antipsychotic
drugs interfere with the neurotransmitter
actions of dopamine, and their antidopaminergic
effects may well account for the diverse
extrapyramidal effects of neuroleptic drugs. The
antidopamine receptor effects of neuroleptic
drugs also influence hypothalamic regulatory
hormones and result in profound changes in the
endocrine system, such as increased secretion of
prolactin.
-
- Although evaluating the efficacy of any drug
is problematic, with psychoactive drugs it is
particularly difficult because of the
limitations of screening and testing methods
used to develop new agents, most of which offer
few advantages over drugs already available for
treatment. The essential characteristics of
human mental disorders cannot be reproduced in
animals. Cognition.. communication, and social
relationships in animals are difficult to
compare with those in humans, and thus,
screening procedures in animals are of limited
utility for the discovery of unique therapeutic
agents. In addition, clinical evaluation of new
drugs is hampered by nonhomogeneity of
diagnostic groups and difficulty in applying
valid, sensitive measurements of the effects of
therapy.
-
- The present review has been prepared on the
basis of our experimental results obtained on
yawning and prolactin with respect to drug
evaluation of new potential antipsychotic
agents.
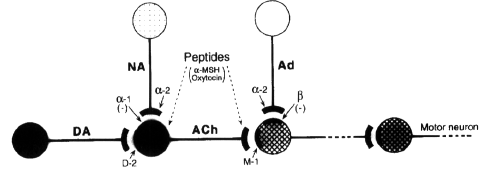 - DA dopamine ; Ach acetylcholine ; NA
noradrenergic ; Ad adrenergic ; M1 muscarinic
recep ; D2 dopamine recep
-
- Behavioral drug evaluation of agents
acting on dopaminergic function
- A major goal of basic science research in
the design of animal models has been to
elucidate the neurochemical basis of
psychotropic disorders in order to define
pathogenesis and to improve treatment. As the
biochemical bases of several abnormal symptoms
are understood, the role of neurotransmitter
systems in the pathogenesis of the disease might
evolve. Because of the difficulty in defining
one animal model that precisely matches all of
the components of human psychosis, this goal may
never be achieved. However, it is feasible to
design an animal model for one facet of a
complex clinical syndrome and thereby analyze
the neurochemical mechanism responsible for the
behavioral abnormality. For analysis of
dopaminergic rnechanisms, behavioral animal
models such as hyperaclivity, stereotypy and
rotation have been used.
-
- Hyperactivity : Results of the
considerable work performed in animals suggest
that increased motor activity (locomotion)
induced by stimulants is related to stimulation
of brain dopaminergic activity. In addition,
biochemical and behavioral findings indicate the
participation of activation at the brain
norepinephrine level as well. Other experiments
have implicated an inhibitory function of
serotonergic systems in controlling motor
activity. Thus, locomotor activity seems to
involve alterations in several brain
neurotransmitter systems and, therefore, may not
be necessarily selective for dopaminergic
mechanisms (1, 2).
-
- Stereotypy : Agents that facilitate
dopaminergic activity, such as apomorphine and
d-amphetamine, can produce stereotypy
(repetitive movements such as sniffing, licking,
biting and gnawing), and neuroleptics, which
block dopaminergic receptors, contrarily reduce
the intensity of this behavior. Pergolide, a
dopamine Dl and D2 receptor agonist, also evokes
stereotyped behavior (3-5). It is also suggested
that amphetamine-induced locomotor activity is
at least partly mediated by dopamine released
from mesolimbic dopamine neurons, whereas the
stereotyped behavior is more closely related to
the activity of nigrostriatal dopamine neurons
(6). Hence, a predominant view at this time is
that excessive dopaminergic activities in the
brain are involved in causing stereotyped
behavior (1).
-
- Rotation (circling, turning) behavior
: In animals with ascending nigrostriatal
dopamine neurons unilaterally destroyed by local
injections of 6-OHDA, d-amphetamine causes
rotation toward the side of the lesion, whereas
apomorphine causes rotation away from it; thus
animals rotate away from the side of the
greatest dopaminergic activity in the striatum.
This appears to be due to the development of
supersensitive dopamine receptors in the
lesioned striatum. Furthermore, bilateral
destruction of mesolimbic dopaminergic nerve
terminals by injection of 6-OHDA into the
nucleus accumbens alters drug-induced rotation
behavior of rats and results in a reduced rate
of rotation in response to d-amphetamine and an
enhanced rate in response to apomorphine (6).
Pergolide, a dopamine receptor agonist, elicits
turning behavior (5, 7).
-
- Yawning
- Dopamine : It has been reported that
apomorphine exerts biphasic effects on behavior,
that is, a decrease of motor activity at low
doses, and stereotypy and hypermotility at high
doses. We administered apomorphine to rats in
order to find a certain modification of
behavior, especially stereotypy, which has been
proposed to be caused by dopamine receptor
stimulation under several experimental
conditions. However, the rats unexpectedly
showed recurrent episodes of yawning with or
without penile erections after injection of low
doses of apomorphine (8). Each yawn was preceded
by grooming or chewing or sometimes by sudden
stretching of the forelimbs. Yawning began
within 5 min after injection and was marked
after 10-20 min. These responses to apomorphine
were most pronounced at a dose of 0.25 mg/kg;
the incidence of yawning at this dose was 87.5%,
with a mean number of 4 yawns in 60 min. At
higher doses, apomorphine induced dose-
dependent stereotypy, characterized by slight
sniffing at 0.5 mg/kg and by continuous licking
and biting at 2.0 mg/kg, as previously reported.
These responses were greatest 20 min after
injection.
-
- Yawning and stereotypy did not appear
simultaneously (8). It was reported that
intraventricular administration of the
cholinergic agents ACTH, MSH or P-LPH, including
their synthetic peptides, elicited yawning
accompanied by sexual excitement, such as penile
erections, in rats (9) and rabbits (l 0, 11).
These agents were also reported to cause yawning
in infant rats (12). The dose-response curve of
yawning to apomorphine showed two peaks at doses
of 0.05 and 0.5 mg/kg (8) i.p., suggesting that
the apomorphine used in this experiment may have
been decomposed since it had been stored for a
fairly long period of time. We then performed a
dose-response run with a new batch of the drug
and obtained a dose-response curve with one peak
at a lower dose of 0.25 mg/kg (Fig. 1) ( 13).
The dopamine receptor agonists bromocriptine
(1-32 mg/kg), piribedil (0.2-5 mg/kg), and 3-PPP
(5-20 mg/kg) also produced dose-dependent
yawning behavior with one peak, dernonstrating
that the incidence of yawning decreases at
higher doses of dopaminergic agents with optimal
peak doses (14), as occurs with apomorphine. The
dopamine receptor agonists thus produce yawning
at lower doses and stereotypy at higher doses
(13-16).
-
- It has also been reported that low doses of
apomorphine preferentially activate presynaptic
dopamine autoreceptors, which results in an
inhibition of dopamine release and consequent
decrease in its synthesis, whereas higher doses
stimulate postsynaptic receptors ( 17. 18).
Accordingly, we first proposed that yawning
elicited by low doses of apomorphine may be due
to activation of presynaptic dopamine
autoreceptors, while stereotypy induced by
higher doses may be attributed to stimulation of
postsynaptic dopamine receptors. However, after
development of the microdialysis method, Stahle
et al. determined apomorphine-induced
extracellular dopamine levels in the rat corpus
striatum and proposed that yawning and
suppression of exploration induced by dopamine
agonists are not related to changes in
extracellular dopamine levels. On the basis of
such findings, they proposed that autoreceptors
are not mediators of behavioral effects of
dopamine receptor agonists and that postsynaptic
receptors mediate agonist-induced yawning (19,
20).
-
- Apomorphine-induced yawning was completely
antagonized by fluphenazine, a dopamine receptor
antagonist (8). In addition, yawning produced by
apomorphine, piribedil and 3-PPP, dopamine
receptor agonists, was also strongly antagonized
after sulpiride (14) and haloperidol (13),
dopamine D2 receptor antagonists (Fig. 2).
-
- Talipexole (B-HT 920), a selective dopamine
D2 receptor agonist, dose-dependently evoked
yawning but did not cause or caused only slight
stereotyped behavior even at larger doses (21,
22). Yawning caused by talipexole was strongly
inhibited by spiperone and YM-0915 1, D2
receptor antagonists, but was unaffected by SCH
23390, a Dl receptor antagonist. On the other
hand, SK&F 38393, a Dl receptor agonist, did
not elicit yawning behavior (21).
-
- There as been substantial evidence that
functional responsiveness of central dopamine
receptors can be altered in response to synaptic
situation. The supersensitivity of post-synaptic
dopamine receptors occured after long-term
impairment of dopamine neural transmission by 6-
hydoxydopamine or reserpine (23, 24). In this
respect, reserpine is known to cause
supersensitivity of dopamine D2 receptors 18 h
or more, but not 5 h, after treatment in rats
(25). Twenty-four hours after treatment with
reserpine, yawning induced by apomorphine,
piribedil and talipexole was markedly
potentiated (8, 26) and was antagonized by
spiperone, a D2 receptor antagonist. Under even
such supersensitive conditions with dopamine
receptors, SCH 23390, a D1 selective agonist,
was not able to cause yawning. These results
suggest that dopamine D2 receptors, but not Dl
receptors, participate in provoking
yawning.
-
- Experiments were also performed to determine
the different properties of the dopamine D2
receptors related to yawning and stereotypy
(22). The incidence of yawning produced by low
doses of talipexole and SND 919, dopamine D2
receptor agonists, was decreased
dose-dependently by SK&F 38393, a dopamine
D1 receptor agonist (Fig. 3). On the other hand,
talipexole and SND 919 at a high dose did not
evoke or evoked only slight stercotypy, but the
incidence of stereotypy by these agents was
increased dose-dependently by SK&F 38393
(Fig. 4). Accordingly, the D2 receptors related
to yawning are more sensitive to dopamine
receptor agonists than those related to
stereotypy. Moreover, concurrent stimulation of
postsynaptic dopamine D1 receptors with D2
receptors reduces the incidence of yawning but
enhances that of stereotypy (22).
- Recent gene cloning studies have
demonstrated the existence of different families
of D1 -like (D A, D1B, D5) and D2-like (D2
long/short, D3, D4) receptors (27). Currently,
considerable interest is focused on dopamine D3
receptors (28). Many antipsychotics display very
high affinity for D3 receptors expressed in
Chinese hamster ovary cells (29). For example,
quinpirole was believed to be a selective
dopamine D2 receptor agonist until recently,
when it was demonstrated to have a 113-fold
greater affinity for D3 receptors than for D2
receptors following discovery of dopamine
receptor subtypes (28). Recently, 7-OH-DPAT was
also identified as a dopamine receptor agonist
having a higher affinity for D3 than for D2, D4
and D1 receptors (30). In addition, the signal
transduction mechanism involved in D3 receptor
responses seems to differ from that of its
closest homologue, the D2 receptor (31).
-
- Both 7-OH-DPAT and quinpirole evoked similar
yawning behavior; the dose-response was
bellshaped wilh a maximal effect at 25 and 100
µg/kg, respectively. These responses were
strongly inhibited by spiperone, a D2 receptor
antagonist (32). In the study on rat serum
prolactin levels, 7-OH-DPAT and quinpirole also
decreased levels dose-dependently (33) at a dose
range similar to those of talipexole and SND
919, D2 receptor agonists (22). The decrease in
prolactin levels induced by both drugs was
antagonized by spiperone (33). Regarding these
results, spiperone has been used as a dopamine
D2 receptor antagonist but may not necessarily
be selective for D2 receptors since Ki values
were 0.069 and 0.61 nM, respectively, for D2 and
D3 receptors (28). It bas also been proposed
that the anterior pituitary is rich in D2
receptors but lacks D3 receptors (28).
Consequently, dopamine D2 receptors seem to be
involved in evoking yawning and decreasing
prolactin release, although proof of the
possible involvement of D3 receptors must await
the results of future experiments.
-
- Acetylcholine
- Physostigmine, an anticholinesterase agent,
and pilocarpine, a direct acetylcholine agonist,
elicited yawning at low doses and chattering at
high doses in rats (Fig. 1) (8). Yawning
behavior was unaffected by mecamylamine, a
nicotinic receptor antagonist, implying that
nicotinic receptors may not be involved (13,
16). In addition, since the behavior was
abolished by scopolamine but not by
methylscopolamine, a peripheral muscarinic
receptor antagonist, it appears that yawning is
mediated by muscarinic receptor activation in
the brain (8).
-
- Furthermore, apomorphine-induced yawning was
strongly inhibited by dopamine receptor
antagonists and scopolamine, but was not
affected by methylscopolamine (8) and
mecamylamine (13). After treatment with
haloperidol, the yawning was practically
eliminated, while with pilocarpine and
physostigmine it was interestingly unchanged
(13, 16). Yawning elicited by talipexole and SND
919 was strongly reduced not only by spiperone
and YM- 09151-2, D2 receptor antagonists, but
also by scopolamine. These results indicate that
a dopaminergic mechanism precedes the
cholinergic one, and doparninergic-cholinergic
activation seems to he Closely involved in
causing yawning behavior (Fig. 2).
-
- M1, M2 and M3 receptors have been proposed
as subtypes of the muscarinic receptor. M1
receptors exist in the brain, in such areas as
the hippocampus, cerebral cortex and striatum.
Several M 1 receptor agonists have been
developed as potential antidementia agents.
(-)-YM 796, a new muscarinic M1 receptor
agonist, induced yawning which was potentiated
by beta-receptor blocking agents. Yawning
produced by YM 796 in combination with pindolol
was inhibited by scopolamine, pirenzepine, and
EEDQ, a M1 receptor antagonist, but not by
spiperone and 4-DAP, a muscarinic M3 receptor
antagonist (34). RS-86, a putative muscarinic M1
receptor agonist, administered
intracerebroventricularly at low doses and
subcutaneously at high doses, also produced
yawning which was antagonized by scopolamine
(35). Accordingly, the muscarinic M1 receptor
seems to participate in evoking yawning.
-
- Noradrenaline, adrenaline
- Apomorphine-induced yawning was increased by
pindolol, propranolol, indenolol, alprenolol and
bucumolol which block the central
beta-adrenoceptors, but not by the peripheral
beta-adrenoceptor antagonists carteolol and
atenolol (Fig. 5) (36). These beta-adrenoceptor
antagonists given alone did not elicit yawning.
Conversely, yawning was inhibited by salbutamol,
a beta-adrenoceptor agonist, without being
affected by prazosin, an alpha-adrenoceptor
antagonist. The combined administration of
SK&F 38393, a dopamine D1 receptor agonist,
and the beta-adrenoceptor antagonists did not
induce yawning (36). Yawning produced by
talipexole and SND 919 was also potentiated by
pindolol, without causing stereotypy (37).
Yawning elicited by either apomorphine or
piribedil in combination with pindoloi was
suppressed by spiperone and YM-09151, dopamine
D2-receptor antagonists, and scopolamine, a
muscarinic receptor antagonist, but not by SCH
23390, a dopamine D1-receptor antagonist.
Physostigmine or pilocarpine-induced yawning was
also enhanced by pindolol and propranolol. This
enhanced yawning was inhibited by scopolamine,
but not by spiperone, YM-09151-2 and SCH 23390.
Since the beta-adrenoceptor blockade facilitates
the occurrence of yawning induced by
dopaminergic and cholinergic agonists, the
central adrenergic neuron systems may take part
in the regulation of yawning responses
(36).
-
- There is evidence that adrenergic neurons,
possessing high
phenylethanolamine-N-methyltransferase activity
which converts noradrenaline to adrenaline,
exist in the brain (.38, 39). Intraperitoneal
injection of tacrine or NIK-247, cholinesterase
inhibitors, induced yawning which was markedly
increased by pretreatment with a
beta-adrenoceptor antagonist, pindolol. Yawning
evoked by tacrine or NIK-247 given alone or in
combination with pindolol was inhibited by
pretreatment with scopolamine, but not by
spiperone. Treatment with tacrine or NIK-247
increased acetylcholine content in the striatum,
but this effect was not enhanced by pindolol
which per se did not affect basal acetylcholine
content (40). Pretreatment with the central
adrenaline synthesis inhibitors LY-78335 and UK-
1187A also increased tacrine-induced yawning
(40). Subcutaneous injection of talipexole
evoked yawning, which was also increased by
pindolol, LY-78335 and UK- 1187A (37). These
receptor antagonists and synthesis inhibitors
per se did not cause yawning responses.
- Since beta-adrenoceptor blockade and
inhibition of adrenaline synthesis similarly
facilitate yawning induced by cholinergic and
dopaminergic agonists, the central adrenergic
neuronal systems seem to be implicated in the
regulation of yawning responses (40). Our
results indicate that adrenergic neuronal
activity inhibits cholinergic but not
dopaminergic activation which is involved in
causing yawning behavior . It is also suggested
that adrenergic neurons interact with
cholinergic neurons, and yawning caused by
cholinergic activation is increased via
adrenergic beta-receptor blockade. In addition,
it has been confirmed that the stimulation of D1
receptors is not involved in the occurrence of
yawning since dopamine D1 receptor stimulants
are not able to evoke yawning even after
treatment with beta-receptor blockers.
-
- Yawning behavior elicited by talipexole was
increased not only by pindolol, a
beta-adrenoceptor antagonist, but also by
prazosin and bunazosin, alpha-adrenoceptor
antagonists. However, the yawning induced by
physostigmine, an anticholinesterase agent, and
pilocarpine, a direct muscarinic receptor
agonist, was increased by pindolol but was
unaffected by prazosin and bunazosin. In
addition, yawning induced by the dopaminergic
agonists, but not by the cholinergic agonists,
was markedly suppressed by ST587, an
alpha1-adrenoceptor agonist. All the yawning
responses to dopaminergic and cholinergic agents
were reduced not only by scopolamine, a
muscannic receptor antagonist, but also by
idazoxan, rauwolscine and yohimbine,
alpha2-adrenoceptor antagonists (41).
-
- Alpha-adrenoceptors have been subclassified
into alpha1 and alpha2 subtypes. It has also
been proposed that alpha2-adrenoceptors are
located on noradrenergic and adrenergic neuronal
pathways, and alpha2-adrenoceptor antagonists
increase both noradrenaline and adrenaline
release via blockade Of alpha2-receptor at
central catecholaminergic nerve terminals (42,
43). Consequently, the noradrenergic neuronal
mechanism appears to interact with dopaminergic
mechanisms and participates via alpha1-receptor
in decreasing the incidence of yawning caused by
dopaminergic agonists without influencing the
behavior induced by cholinergic agonists. It is
also suggested that the stimulation of
noradrenergic and adrenergic mechanisms induced
by an increase in noradrenaline and adrenaline
release resulting from presynaptic
alpha2-receptor blockade might result in
inhibition of yawning evoked by both
dopaminergic and cholinergic activation (Fig. 2)
(41).
-
- Serotonin
- The yawning responses to apomorphine,
piribedil and talipexole, dopamine receptor
agonists, were markedly increased by
pretreatment with reserpine without eliciting
stereotypy (26). Piribedil-induced yawning was
markedly inhibited after treatment with
fluphenazine, scopolamine and methysergide (44).
Yawning induced by apomorphine and talipexole
was also increased by the serotonin synthesis
inhibitor, p-chlorophenylalanine (PCPA), but was
not affected by a-methyl-p-tyrosine (alpha-MT),
implying that depletion of serotonin plays an
important role in potentiation of yawning (26).
It was later reported that apomorphine-elicited
yawning was enhanced by pretreatment with PCPA
or the serotonergic neurotoxin,
5,7-dihydroxytryptamine, and was contrarily
reduced by the serotonin precursor,
5-hydroxytryptophan (45). In fact, serotonin is
found in relatively high concentrations in the
rat striatum (46, 47), one of the sites of
action of dopamine receptor agonists in yawning
(45, 48). Various lines of evidence have shown
that the origin of serotonergic neurons in the
striatum is the dorsal raphe (49), and the
inhibitory serotonin receptors are located on
terminals of dopaminergic neurons in the
striatum (150-52).
-
- Lesioning of the raphe nucleus which reduces
serotonin levels in the forebrain has been
reported to cause an increase in dopamine
release (53). The yawning evoked by combined
administration of talipexole and PCPA was
completely inhibited following spiperone or
scopolamine (26). Therefore, treatment with PCPA
may evoke an increased release of dopamine which
plays a facilitatory role in the occurrence of
yawning. Thus, it is assumed that the
potentiation by reserpine or PCPA of yawning
induced by dopamine receptor agonists involves
decreases in serotonergic neuronal
activity.
- Intraventricular injection of
a-melanocyte-stimulating hormone (alpha-MSH)
elicited not only yawning-stretching syndrome
but also 'wet dog' body shaking. Yawning was
synchronized with stretching in almost all
cases. The alpha-MSH-induced yawningstretching
syndrome was blocked by scopolamine,
apomorphine, fluphenazine and methysergide.
Therefore, reciprocal balance of serotonergic
activation, dopaminergic inhibition and
cholinergic activation is involved in yawning
produced by (alpha-MSH (44).
-
- Neuropeptides
- Central administration of
adrenocorticotropic hormone (ACTH) was reported
to cause yawning behavior (54, 55). Wood et al.
(56) have proposed that the septal-hippocampal
cholinergic neurons are necessary to elicit a
specific stretching-yawning syndrome following
ACTH or alpha MSH.
-
- The peptide oxytocin also elicited yawning.
The yawning response to oxytocin was markedly
increased by pretreatment with an
beta-adrenoceptor antagonist, pindolol (20
mg/kg), which per se did not elicit yawning. The
yawning induced by oxytocin (50 ng/rat, i.c.v.)
plus pindolol, but not that by alpha-MSH (20
µg/rat, i.c.v.) plus pindolol, was
inhibited by
[d(CH2)5,Tyr(Me)2,Om8l-vasotocin (100
ng/rat, i.c.v.), an oxytocin receptor
antagonist. Yawning induced by oxytocin or
alpha-MSH administered in combination with
pindolol was inhibited by scopolamine (0.5
µng/kg, s.c.), a muscarinic receptor
antagonist, without being affected by spiperone
(0.5 µng/kg, s.c.). a dopamine D2 receptor
antagonist. Thus, yawning produced by the
neuropeptides, oxytocin and alpha-MSH, is
modulated by beta-adrenoceptor activity in an
inhibitory manner similar to that of muscarinic
M1 receptor agonists, and involves cholinergic,
but not doparninergic, activation (35). These
results are in agreement with the previous
proposal that the expression of yawning induced
by dopaminergic agonists involves
dopamine-oxytocin, but not oxytocin- dopamine,
neuronal linkage (57). Yawning evoked by an
alpha-MSH-related peptide, ACTH, which was
unaffected by oxytocin receptor antagonists, was
also reported to be prevented by cholinergic
receptor antagonists (58).
-
- Our previous results have indicated that
none of the behavioral responses to alpha-MSH,
such as yawning, stretching and body shaking,
are associated with changes in the activities of
the nigrostriatal, mesolimbic,
tuberoinfundibular, or tuberohypophyseal
dopaminergic neurons (44, 59), and that
alpha-MSH-induced yawning is decreased by
administration of cholinergic receptor
antagonists (44). Yawning evoked by alpha-MSH
administered after pindolol was antagonized by
scopolamine but not by spiperone. From such
findings, the oxytocin- and alpha-MSH-induced
yawning responses appear to involve cholinergic
but not dopaminergic activation. According to
our proposal that dopaminergic cholinergic
activation is involved as a common principal
mechanism in causing yawning, the peptidergic
mechanisms appear to be positioned between the
dopaminergic and cholinergic neuronal system.
Moreover, the present results also indicate that
beta-adrenoceptors link to cholinergic neurons
in the yawn-inducing neuronal mechanism and
thereby play an inhibitory role in the
modulation of such behavior.Thus, the
neuropeptides, oxytocin and alpha-MSH, produce
yawning via activation of cholinergic
mechanisms, and beta-adrenoceptors are involved
in the regulation of this yawning (Fig. 2).
-
- Sites in the
brain
- We have investigated possible areas in the
brain where a doparninergic-cholinergic neuron
link may be involved with the incidence of
yawning. Various lines of evidence suggest that
the nigrostriatal dopaminergic neurons interact
with the striatal cholinergic neurons, while a
dopaminergic-cholinergic link is lacking in the
mesolimbic area such as the nucleus accumbens
and olfactory tubercle. Other evidence suggests
that the mesoseptal dopaminergic neurons play a
role in the control of the septal-hippocampal
cholinergic neurons. The septal-hippocampal
cholinergic neurons have been proposed to be
necessary to elicit a specific
stretching-yawning syndrome following a-MSH,
since intraventricular injection of alpha-MSH
caused yawning and also increased acetylcholine
turnover rate in the rat hippocampus (60).
-
- When dopamine receptor agonists such as
apomorphine, piribedil and 3-PPP are bilaterally
injected into the striatum and septum at smaller
doses, yawning is markedly evoked (14).
Consequently, the striatal and septal
dopaminergic system may be related to the
occurrence of yawning behavior, although other
possible sites in the brain are still
unclear.
-
- Prolactin
- Dopamine in the brain is involved in the
regulation of hormone secretion. The
tuberoinfundibular dopamine neuron, which
originates in the arcuate and periventricular
nuclei of the hypothalamus and projects to the
external layer of the median eminence, is
especially known to mainly regulate prolactin
secretion from the anterior pituitary. Dopamine
released from the median eminence into the
hypophyseal portal vessels reaches the anterior
pituitary and tonically suppresses prolactin
secretion by acting on D2 receptor at
prolactin-secreting cells which are endowed with
inhibitory D2 receptor (6 1 ). In additiion, D2
receptors in lactotroph cells are expressed by
the same DNA found in areas of the brain such as
the striatum, cerebral cortex and nucleus
accumbens (62). There is little evidence that
tuberoinfundibular dopamine neuron of the
hypothalamus is endowed with autoreceptors
regulating activity of neurons and release of
dopamine from the nerve terminals in the median
eminence (63). Consequently, with regard to
regulation of prolactin release, dopaminergic
drugs administered in vivo appear to act
preferentially on D2 receptors of the pituitary
lactotroph cells rather than autoreceptors in
the hypothalamus.
- Consequently, in addition to observing
yawning behavior, we also determined prolactin
release from isolated rat pituitary slices and
serum prolactin levels in male rats.
- Pergolide, a dopamine D1 and D2 receptor
agonist, decreased plasma prolactin levels (5),
and perphenazine, a dopamine receptor
antagonist, elevated them (64). 3-PPP,
talipexole and SND 919, dopamine D2 receptor
agonists, at respective yawninducing doses also
caused a reduction in both the basal prolactin
levels and ct-methyl-p-tyrosineinduced
hyperprolactinemia (21). The high serum
prolactin levels produced by daily treatment
with estradiol were also reduced by talipexole
and SND 919 in a dose-dependent fashion. These
inhibitory effects were blocked by concornitant
administration of YM09151-2, a dopamine D2
receptor antagonist (65). As described above,
7-OH-DPAT and quinpirole, dopamine D3 receptor
agonists, also dose-dependently reduced
prolactin levels and the reductions were
antagonized by spiperone, a dopamine D2 receptor
antagonist, presumably because the anterior
pituitary is rich in D2 receptors but lacks D3
receptors (33).
- YAWNING FOR
PRECLINICAL DRUG EVALUATION
- Talipexole (B-HT 920)
- Talipexole was developed in Europe. On motor
activity, the agent decreases locomotion but
increases the activity when postsynaptic DA
receptors become supersensitive 12-48 h after
reserpine administration in mice and rats.
Talipexole, at a wide range of doses, does not
cause stereotyped behavior. The drug does not
evoke rotation behavior but does cause the
behavior only when supersensitive DA receptors
exist after treatment with 6-OHdopamine. On the
basis of such behavioral, electrophysiological
and biochemical studies, it was proposed that
talipexole, because it does not cause stereotypy
and rotation, is a presynaptic dopamine
D2-autoreceptor agonist with or without minor
actions on postsynaptic dopamine D2 receptors.
Therefore, it was proposed that talipexole may
be therapeutically valuable in diseases presumed
to be accompanied by a predominance of brain
dopamine activity, such as Huntington's disease,
mania and schizophrenia (66-68.).
-
- In our studies, talipexole markedly induced
yawning with bell-shaped dose responses at
smaller doses but did not cause or caused only
slight stereotyped behavior even at higher doses
(21, 22). SND 919, having a similar chemical
structure as talipexole, also caused yawning
with bell-shaped dose responses at smaller doses
and caused slight stereotypy (21, 22). The
yawning caused by talipexole and SND 919 was
inhibited by spiperone and YM-09151-2. dopamine
D2 receptor antagonists, and scopolamine but was
unaffected by SCH 23390, a dopamine D1 receptor
antagonist (21), showing that talipexole and SND
919 are postsynaptic dopamine D2 receptor
agonists.
-
- As lactotroph dopamine receptors are more
similar to dopamine autoreceptors than to
postsynaptic dopamine receptors in the brain
(68), we also studied effects on prolactin
release. Talipexole and SND 919 dose-dependently
decreased basal prolactin levels in rats. The
decreasing effect of both drugs was marked in
(i-methyl-p-tyrosine-induced hyperprolactinemia
(21). We suggest that talipexole and SND 919
exert selective agonistic activities for
specific dopamine D2 receptors which are related
to causing yawning, but not to stereotypy and
rotation, and that both drugs have a high
affinity for dopamine receptor agonists similar
to that of the pituitary lactotroph dopamine D2
receptors. Finally, talipexole was recognized as
a full agonist at both pre- and postsynaptic D2
dopamine receptors (69-71).
-
- Clinical evaluation oftalipexole in Europe
and a pilotstudy in Japan for the treatment of
schizophrenia has already begun on the basis of
its possible presynaptic dopamine autoreceptor
agonist action without exerting postsynaptic
action. These clinical studies have shown that
the drug is effective at smaller doses,
presumably because of presynaptic agonistic
action, but with increased doses efficacy
disappears and patients' symptoms are often
aggravated, probably because of postsynaptic
dopamine D2 receptor stimulation. Based on our
experimental results on yawning and prolactin
release showing talipexole to be a postsynaptic
dopamine D2 receptor agonist, clinical trials
were focused on the drug as an antiparkinsonian.
Talipexole exhibits good efficacy with less
gastrointestinal side effects, such as nausea
and vomiting, and was recently approved in Japan
for the treatment of Parkinson's.
-
- Aripiprazole (OPC-14597)
- Aripiprazole inhibited reserpine- and
gamma-butyrolactone-induced increase in tyrosine
hydroxylase activity in the mouse and rat brain
and the effects were completely antagonized by
haloperidol. Aripiprazole, unlike apomorphine,
did not evoke postsynaptic DA
receptor-stimulating behavioral signs such as
hyperlocomotion in reserpinized mice and
contralateral rotation in rats with unilateral
striatal 6-hydroxydopamine lesions. The agent
inhibited apomorphine-induced postsynaptic
behavioral changes such as stereotypy and
hyperlocomotion in mice and rats and rotation in
rats with unilateral striatal lesions by kainic
acid. From these results, aripiprazole was
proposed to be a unique antipsychotic drug
candidate with DA autoreceptor agonistic and
postsynaptic D2 receptor antagonistic activity
(72).
-
- In our studies (73), aripiprazole did not
cause hyperlocomotion or stereotypy in rats and
did not evoke rotation behavior in
6-hydroxydopaminepretreated rats. In addition,
apomorphine-induced stereotypy was antagonized
by haloperidol and aripiprazole. showing that
aripiprazole exhibits the profile of a
postsynaptic D2 receptor antagonist. However,
the experiments on yawning (73) demonstrated
that aripiprazole dose-dependently induced the
behavior to a certain extent at doses of 0.21-5
nigikg with significance at 5 mg/kg. The
incidence or yawning was potentiated by
pretreatment with beta-receptor antagonists and
reserpine, as seen also with dopamine D2
receptor agonists. On the other hand, yawning
produced by apomorphine was antagonized by
aripiprazole. Thus, aripiprazole exerts only
antagonistic action on stereotypy and rotation
and partial agonistic action on yawning in
rats.
-
- Prolactin release from the isolated rat
anterior pituitary was dose-dependently
decreased by aripiprazole with weaker potency
than that of talipexole, a D2 receptor full
agonist (74). The decrease by aripiprazole was
completely antagonized by haloperidol. Morcover,
aripiprazole antagonized the inhibition of
prolactin release elicited by talipexole. In in
vivo studies (Fig. 6) (74), haloperidol
increased serum prolactin levels by 8-fold above
the basai level, whereas talipexole decreased
them to 49% of the basal level. Aripiprazole
dose-dependently increased the levels by 2-fold.
Because of increased biosynthesis and release of
prolactin in lactotroph cells, estradiol
treatment in rats caused elevated serum
prolactin levels which stimulated the activities
of the tuberoinfundibular dopamine neuron and
increased dopamine concentrations in the
hypophyseal portal blood (75, 76).
Hyperprolactinemia induced by estrogen was
inhibited by talipexole and enhanced by
haloperidol and aripiprazole (74). In contrast,
reserpine is known to decrease dopamine levels
in DA nerve terminals and in the rat pituitarv
portal blood and cause supersensitivity of D2
receptor 18 h or more, but not 5 h. after
treatment in rats (76). The hyperprolactinernia
caused 5 h after reserpine was inhibited by
talipexole and aripiprazole and elevated by
haloperidol (74). Thus, our results obtained
from effects on yawning and prolactin release
interestingly indicate that aripiprazole bas a
mixed agonist/antagonist profile at D2 receptors
and exerts an antagonistic or agonistic action
depending on preexisting tone of dopaminergic
neuronal activities.
-
- Antipsychotic agents such as haloperidol are
known to exert their therapeutic effect on
schizophrenia through blockade of dopamine D2
receptors in the mesolimbic and mesocortical
dopamine neurons but simultaneously cause
undesirable extrapyramidal and endocrinological
side effects, e.g., hyperprolactinemia, due to
blockade of D2 receptors in the striaturn and
anterior pituitary (77). Aripiprazole acts as an
antagonist against the excess DA release at
over-acting synapses in the brain of psychotic
patients, but a low intrinsic activity of the
drug can counteract a full blockade of D2
receptors in the striatum and pituitary.
Therefore, aripiprazole appears to be a
potential antipsycholic drug that does not cause
severe side effects, such as extrapyramidal
symptoms and hyperprolactinemia. In fact, it has
been suggested from a clinical phase II study in
Japan that aripiprazole is effective in the
treatment of both negative and positive symptoms
in schizophrenic patients without causing severe
extrapyramidal side effects (78). A large-scale
clinical study is now in progress in both Japan
and the US
- .
- Partial agonists on dopamine
receptors
- Several compounds were proposed to be
partial agonists on dopamine receptors, since
they produced some stereotypy and rotation but
antagonized the behavior evoked by dopamine
receptor agonists such as apomorphine. However,
they induced marked yawning and slightly
antagonized apomorphine-induced yawning in our
study, indicating that these partial agonists
have a relatively strong agonistic and weak
antagonistic profile. Clinical trials with these
agents have been discontinued because of
frequent aggravation of'symptoms in
schizophrenic patients, probably because
oftheirdominant agonistic effects.
- Our experimental results of talipexole,
aripiprazole and potential partial agonists
obtained from animal studies on awning and
prolactin release, but not those seen with
studies on locomotion, stereotypy and rotation,
coincide with the clinical effects of potential
drugs.
-
- Potential nootropic agents acting on
cholinergic function
- Tacrine, 9-amino-1,2,3,4-tetrahydroacridine,
a potent, centrally acting cholinesterase
inhibitor (79), is available in the US as an
antidementia agent. NIK-247,
9-amino-2,3,5,6,7,8-hexahydro-IH-cyclopenta-(6)-quinoline
monohydrate HCI, developed in Japan as
acholinesterase inhibitor, improves cognitive
functions at different phases of the learning
and memory process in rats (80). Both agents
dose-dependently induced yawning which was
markedly increased by pretreatment with a
beta-adrenoceptor antagonist or adrenaline
synthesis inhibitor, and the yawning produced by
these agents was inhibited by scopolamine
without being affected by mecamylamine or
spiperone. These agents also increased
acetylcholine content in the striatum (Fig.
7)(40).
- Muscarinic M1 receptor agonists have also
been developed as possible antidementia agents.
A new muscarinic receptor agonist, YM 796, has
high affinity for M1 receptor. In our studies
(34), YM 796 elicited yawning behavior which was
potentiated by beta-adrenoreceptor antagonist
and inhibited by scopolamine and pirenzepine, as
well as EEDQ, M1 receptor antagonists, but not
by spiperone, a dopamine D2 receptor antagonist,
and 4-DAMP a muscarinic M3 receptor antagonist
(81). Thus, it is possible to assess the
cholinergic activation in the brain whether or
not these agents cause yawning behavior.
- Discrimination between central and
peripheral betaadrenoceptor blocking
agents
- Yawning produced by dopamine receptor
agonist was potentiated by central
beta-adrenoceptor antagonist which blocked
beta-adrenoceptor in the brain after passing
through the blood-brain barrier, but not by
peripheral antagonists (Fig. 5). The yawning
induced by pilocarpine and physostigmine was
also increased by the central beta-receptor
antagonist (36). The behavior evoked by a
neuropeptide, oxytocin and
alpha-melanocyte-stimulating hormone was also
potentiated after the antagonists (35). Thus, it
is possible to functionally discriminate between
central and peripheral beta-adrenoceptor
antagonists.
-
- CONCLUSIONS
- Dopaminergic agents cause yawning at smaller
doses and stereotypy at larger doses. The
dopamine D2 receptors related to yawning are
thus more sensitive to dopamine receptor
agonists than those related to stereotypy. In
addition, yawning is more selective for D2
receptor activation than stereotypy and
rotation. Cholinergic agents also elicit yawning
and the doparninergic-cholinergic neuronal link
appears to be principally involved. The results
obtained from yawning studies, but not those on
stereotypy and rotation, are compatible with the
clinical effects of potential antipsychotic and
antiparkinsonian agents.
-
- -Fugikawa
M; Yamada K; Nagashima M; Furukawa T
Involvement of beta-adrenoreceptors in
regulation of the yawning induced by
neuropeptides; oxytocin and alpha-melanocytes
stimuling hormone in rats. Pharmacol Biochem
Behav 1995; 50; 339-343
- -Furukawa
T Yawning behavior for preclinical drug
evaluation Meth Find Exp Clin Phamacol 1996; 18;
2; 141-155
- -Kimura H;
Yamada K; Nagashima M; Matsumoto S Role of
adrenergic neuronal activity in the yawning
induced by tacrine and NIK-247 in rats.Pharmacol
Biochem Behav 1992; 43; 4; 985-91
- -Kimura
H; Yamada K; Nagashima M; Furukawa T
Involvement of catecholamine receptor activities
in modulating the incidence of yawning in
rats.Pharmacol Biochem Behav 1996; 53(; 4;
1017-21
- Ogura
H, Kosasa T, Kuriya Y, Yamanishi Y Central
and peripheral activity of cholinesterase
inhibitors as revealed by yawning and
fasciculation in rats. Eur J Pharmacol. 2001;
415; 2-3; 157-64
- -Matsumoto
S, Yamada K, Nagashima M, Matsuo N, Shirakawa K,
Furukawa T Potentiation by serotonergic
inhibition of yawning induced by dopamine
receptor agonists in rats.Pharmacol Biochem
Behav 1989; 32; 3; 815-8
- -Serra
G , Collu M and Gessa GL Yawning is elicited
by D2 dopamine agonists but is blocked by D1
antagonist Psychopharmacology 1987; 91;
330-337
- -Serra G,
Gessa GL Hypophysectomy prevents yawning and
penile erection but not hypomotility induced by
apomorphine Pharmacology Biochemistry &
Behavior 1983; 19; 917-919
- -Serra
G et al Cycloheximide prevents apomporphine
induced yawning, penile erection and genital
grooming in rats European Journal of
Pharmacology1983; 86; 279-282
- -Kostrzewa RM and R
Brus Is dopamine-agonist induced yawning
behavior a D3 mediated event? Life Sci 1991; 48;
26; 129
- -Ushijima I, Mizuki
Y, Yamada M Multifocal sites of action
involved in dopaminergic-cholinergic neuronal
interactions in yawning Psychopharmacology
(Berl) 1988; 95; 34-7
- -Ushijima
I et al, Muscarinic and nicotinic effects on
yawning and tongue protruding in the rat
Pharmacol Biochem Behavior 1984; 21;
297-300
- -Ushijima
et al modification of apomorphine,
physiostigmine and pilocarpine induced yawning
after long term treatment with neuroleptic or
cholinergic agents Arch Int Pharmacodyn 1984;
271; 180-188
- -Ushijima
I et al Characteristics of yawning behavior
induced by apomorphine, physostigmine and
pilocarpine Arch Int Pharmacodyn 1985; 273;
196-201
- -Ushijima,
I., Y. Mizuki, et al. Behavioral effects of
dilazep on cholinergic, dopaminergic, and
purinergic systems in the rat. Pharmacol Biochem
Behav 1992;43(3): 673-676.
- -Yamada
K, Furukawa T Direct evidence for
involvement of dopaminergic inhibition and
cholinergic activation in yawning
Psychopharmacology 1980; 67; 39-43
- -Yamada
K, Furukawa T The yawning elicited by
alpha-melanocyte-stimulating hormone involves
serotonergic -dopaminergic - cholinergic neuron
link in rats Naunyn-Schmiedeberg's Arch
Pharmacol 1981; 316; 155 -160
- -Yamada
K et al Involvement of septal and striatal
dopamine D2 receptors in yawning behavior in
rats Psychopharmacology 1986; 90; 9-13
- -Yamada
K et al Possible involvement of differing
classes of dopamine d2 receptors in yawning and
stereotypy in rats Psychopharmacology 1990; 100;
141-144
- -Yamada
K, Furukawa T Behavioral studies on central
dopaminergic neurons. especially jumping,
stretching, body shaking and yawning behavior J
PharmacoBio dynamics 1980; 3; S16-S18
- -Yamada
K, Matsumoto S, Nagashima M, Kumagai M, Matsuo
N, Furukawa T Stimulatory effects of
beta-adrenoceptor blockers on the yawning
induced by dopaminergic and cholinergic agonists
in rats. Japanese J Pharmacology 1987; 43;
supp53p
- -Yamada
K, S Matsumoto, M Nagashima, K Shirakawa, T
Furukawa Potentiation of yawning responses
to the dopamine receptor agonists B-HT 920 and
SND 919 by pindolol in the rat J Neural Transm
[GenSect] 1990; 79; 19-24
|


