- Abstract
-
- Yawning is a multifunctional behavior with a
role in social communication. In Old World
monkeys, the "tension yawn" is often used as a
threat, allowing individuals to completely
expose their canines.
-
- To explore the role of this phenomenon, the
authors selected 2 closely related
species-Japanese macaques (Macaca fuscata) and
Tonkean macaques (M. tonkeana)-which differ
primarily in terms of their tolerance levels.
Japanese macaques are classified as despotic;
Tonkean macaques are classified as tolerant.
Both species live in multimale-multifemale
societies, show a high level of sexual
dimorphism, and have comparable yawning
repertoires that include displaying a covered
teeth yawn and an uncovered gums yawn.
-
- They found comparable baseline frequencies
of the 2 yawning types and a similar
distribution of these behaviors according to sex
(males yawned more frequently than females).
This morphological homogeneity permitted us to
evaluate potential differences in the meaning of
yawning as a function of social tension,
aggressive contexts, and dominance hierarchy.
Divergent social styles determine a functional
dichotomy in the use of the covered teeth yawn
and the uncovered gums yawn. The covered teeth
yawn is not susceptible to social and
environmental stimuli and seems to be a form of
yawning mostly linked to the physiology of the
sleep-wake cycle.
-
- However, the uncovered gums yawn is
modulated according to different social
contexts, and its use could be favored by
natural selection, especially in tolerant
species, which apparently require more elaborate
forms of social communication.
- -Demuru
E, Palagi E. In Bonobos Yawn Contagion Is
Higher among Kin and Friends. PLoS One. 2012;
7(11): e49613
- -Leone A,
Mignini M, Mancini G, Palagi E. Aggression
does not increase friendly contacts among
bystanders in geladas (Theropithecus gelada)
Primates. 2010;51(4):299-305
- -Leone A,
Ferrari PF, Palagi E. Different yawns,
different functions? Testing social hypotheses
on spontaneous yawning in Theropithecus gelada.
Scientific Reports 2014;4;4010
- -Norscia
I, Palagi E. Yawn Contagion and Empathy in
Homo sapiens. PLoS ONE. 2011;6(12): e28472
- -Norscia
I, Demuru E, Palagi E. She more than he:
gender bias supports the empathic nature of yawn
contagion in Homo sapiens. R. Soc. open sci.
2016:3:150459.
http://dx.doi.org/10.1098/rsos.150459
- -Palagi
E, Leone A, Mancini G, Ferrari PF.
Contagious yawning in gelada baboons as a
possible expression of empathy. Proc Natl Acad
Sci USA. 2009;106(46):19262-19267
- -Palagi
E, Norscia I, Demuru E. Yawn contagion in
humans and bonobos: emotional affinity matters
more than species PeerJ 2:e519
- -Zannella A,
Norscia I, Stanyon R, Palagi E. Testing
Yawning Hypotheses in Wild Populations of Two
Strepsirrhine Species: Propithecus Verreauxi and
Lemur Catta. Am J Primatol.
2015;77(11):1207-1215
- - Zannella
A, Stanyon R, Palagi E. Yawning and Social
Styles: Different Functions in Tolerant and
Despotic Macaques (Macaca tonkeana and Macaca
fuscata). J Comp Psychol.
2017;131(3):179-188
- -Zannella A,
Stanyon R, Maglieri V, Palagi E. Not all
yawns tell the same story: The case of Tonkean
macaques. Am J Primatol. 2021;83(7):e23263.
- Yawning is a widespread behavior documented
in most vertebrate species (Deputte, 1994), and
it is easily recognizable due to its
plesiomorphic nature (an ancient character
shared by several taxa; Provine, 1996). A yawn
is characterized by a deep inspiration, followed
by a lengthy, forceful expiration with
simultaneous contraction of many skeletal muscle
groups (Darwin, 1872). Recently, authors have
focused on the neurology and physiology of
yawning. Some possible explanations have been
suggested for this behavior, including
respiration, circulation, arousal, brain
oxygenation, thermoregulation, and the
sleep&endash;wake cycle (Gallup, 2014; Giganti
& Zilli, 2011; Matikainen & Elo, 2008;
Provine, 1996). Moreover, the neurological
complexity of yawning is implied because it also
appears to be driven by social complexity
(Campbell & De Waal, 2011; Guggisberg,
Mathis, Schnider, & Hess, 2010; Provine,
1997). Primates have the largest and most
variable yawning repertoire among mammals, most
likely due to their neurological, social, and
morphological complexity (Gallup, Church, &
Pelegrino, 2016).
-
- In sexually dimorphic primate species, males
typically have larger canines than females and
also yawn more frequently (Altmann, 1967).
"Threat yawns" allow males to show their weapons
(i.e., long canine teeth) during both intragroup
ranking competition and intergroup territorial
defense (Macaca nigra, Hadidian, 1980; M.
fascicularis, Deputte, 1994; Troisi, Aureli,
Schino, Rinaldi, & de Angelis, 1990;
Theropithecus gelada, Leone, Ferrari, &
Palagi, 2014). Altmann (1967) suggested that
adolescent baboons learn to appropriately direct
threat yawns toward conspecifics. Thus, yawns
can acquire a communicative function to regulate
social relationships, even if yawns are not
always under voluntary control. Anderson and
Wunderlich (1988) attempted to demonstrate that
food reinforcement led to a certain degree of
"voluntary" control of yawning in M. tonkeana
males. Covered and uncovered tooth yawns in
chimpanzees may indicate either some degree of
voluntary control or a different arousal in the
activation of facial movements (Vick &
Paukner, 2010). However, it is clear that yawns
can be modulated according to context, thus
acquiring different meanings (Vick &
Paukner, 2010). T. gelada displays three
different morphologies of yawning, and only the
uncovered gum yawns (the most intense version of
the motor pattern) are displayed during tense,
agonistic situations (Leone et al., 2014).
Yawning can also be triggered by environmental
and social stressors (Liang, Grace, Tompkins,
& Anderson, 2015; Schino, Maestripieri,
Scucchi, & Turillazzi, 1990). For this
reason, yawning can also be categorized as a
displacement behavioral pattern similar to
scratching (a well-known index of anxiety in
primates; Troisi, 2002). Moreover, yawning
response latency can be environmentally
modulated. It was shown that under laboratory
conditions, perturbing stimuli (e.g., electric
shocks) caused an increase in yawning frequency
after an interval of 20 to 40 min (Miller,
Gallup, Vogel, Vicario, & Clark, 2012;
Moyaho & Valencia, 2002). In the wild,
perturbing stimuli (i.e., predatory attacks and
intragroup aggression) may provoke a faster
yawning response (Zannella, Norscia, Stanyon,
& Palagi, 2015).
-
- The social role of yawning is effectively
demonstrated by its infective nature as reported
in both human and nonhuman animals (Campbell
& De Waal, 2011; Demuru & Palagi, 2012;
Norscia, Demuru, & Palagi, 2016; Palagi,
Leone, Mancini, & Ferrari, 2009; Romero,
Konno, & Hasegawa, 2013). In humans,
contagious yawning is easily triggered by
seeing, hearing, reading, or simply thinking
about another individual yawning (Norscia &
Palagi, 2011; Provine, 2005). Yawn contagion
increases with the level of bonding between
individuals (Palagi, Norscia, & Demuru,
2014), suggesting a link between yawning and
social variables. These data suggest that
yawning is a multifunctional behavior whose
meaning can vary according to social contexts.
Primates, due to their large social variability
both within and between species, provide
promising models to explore the multifaceted
phenomena of social yawning.
-
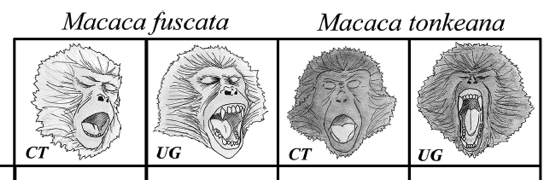
- To investigate the social modulation of
yawning, we selected M. fuscata (Japanese
macaques) and M. tonkeana (Tonkean macaques),
two closely related species (Delson, 1980;
Eudey, 1981; Hoelzer & Melnick, 1996) with
comparable levels of sexual dimorphism. Both
species present two variant displays of yawning:
covered teeth (teeth remain covered also in the
correspondence of the acme phase) and uncovered
gums (teeth and gums exposed along with the
duration of the acme phase). The species
belonging to the genus Macaca can be arranged
along a gradient of social style ranging from
more intolerant (despotic, Grade 1) to more
tolerant (egalitarian, Grade 4; Matsumura, 1998;
Thierry, 2000). These differences in social
styles influence a wide range of behaviors,
including aggressive and affiliative patterns,
dominance relationships, and nepotism (Aureli,
Das, & Veenema, 1997; De Waal &
Luttrell, 1989; Petit, Abegg, & Thierry,
1997; Thierry, 1985). Despotic species such as
Japanese macaques with low levels of social
tolerance have a strong, kin-centric power
asymmetry between dominants and subordinates
(Aureli et al., 1997; Kurland, 1977; Kutsukake
& Castles, 2001). In contrast, egalitarian
species, such as Tonkean macaques, have
relationships that are only moderately
influenced by social ranking and kinship. The
repertoire of formal submissive patterns is
reduced and the proportion of friendly
interactions is high even among unrelated
subjects (Butovskaya, 2004; Butovskaya &
Kozintsev, 1996; Preuschoft & van Hooff,
1995).
-
- The tolerance degree of a given species
relates to how the control over resources is
distributed in social communities (Flack &
De Waal, 2004; Vehrencamp, 1983), which has a
strong impact on their communicative repertoire
(social intelligence hypothesis; Whiten &
van Schaik, 2007). In tolerant societies,
interactions among group members are
characterized by high variability independently
from rank differences and kinship (Butovskaya,
2004; Freeberg, Dunbar, & Ord, 2012). The
high degree of freedom characterizing these
species creates a high level of uncertainty
arising from such interactions (Butovskaya,
2004; Flack & De Waal, 2004). For this
reason, in tolerant social systems, individuals
must rely on greater and more sophisticated
communicative skills in comparison to despotic
species (Flack & De Waal, 2004;
Maestripieri, 1995; Preuschoft, Paul, &
Kuester, 1998; Scopa & Palagi, 2016; Whiten
& van Schaik, 2007).
-
- If yawning is linked, at least in part, to
species-specific communicative abilities, we
expect that, in tolerant societies, yawning can
acquire different functions according to its
different morphologies.
-
- Discussion
- Due to their social complexity and
variability, the species of the genus Macaca are
excellent models to study the multifunctional
nature of social yawning. Japanese and Tonkean
macaques share a common yawning
repertoire&emdash; both species showed covered
teeth and uncovered gum yawns. This
morphological homogeneity permitted us to
evaluate potential differences in the meaning of
yawning depending on different levels of
tolerance. The Japanese macaque had a steeper
dominance hierarchy when compared to the Tonkean
macaque. We also found that both species showed
similar baseline frequencies of yawning, with
males displaying the uncovered gum yawn more
frequently than females. Even though the two
study groups had different sample sizes, all
these findings suggest that they were still
suitable for an accurate comparison (see also
Ciani, Dall'Olio, Stanyon, & Palagi, 2012).
Our findings support the dimorphism hypothesis.
In all primate species in which yawning
frequency significantly differs between sexes,
males always yawn more than females (Altmann,
1967; Bertrand, 1969; Dixson, 2015; Goy &
Resko, 1972; Hadidian, 1980; Hall & DeVore,
1965; Leone et al., 2014; Redican, 1975; Troisi
et al., 1990). This difference is related to the
sexual dimorphism in canine size: The larger
size of canines may make male yawns a more
effective intimidating signal. This hypothesis
is strengthened by the fact that primate
species, which do not show sexual dimorphism in
canine size, have no gender difference in the
baseline frequency of yawning (Lemur catta and
Propithecus verreauxi, Zannella et al., 2015;
Pan paniscus, Demuru & Palagi, 2012; Pan
troglodytes, Vick & Paukner, 2010; Homo
sapiens, Norscia et al., 2016; Schino &
Aureli, 1989).
-
- A functional dichotomy in the use of this
display became clear when we compared different
yawning morphologies in our two study species.
No statistical difference was found in the
frequencies of covered teeth yawns. However, the
two species strongly differed in the performance
of uncovered gum yawns, with Tonkean macaques
exhibiting the highest frequencies of this
behavior, which was mainly performed during
highly tense situations such as feeding (the
around-food condition). Covered teeth yawns were
more frequent in the relaxed condition.
-
- No differences were found between these two
yawn types in Japanese macaques. The presence of
a functional dichotomy in the use of yawning
morphologies in the tolerant species but not in
the despotic species supports the communicative
redundancy hypothesis, which predicts that
tolerant species will tend to show a larger
communicative repertoire (Dobson, 2012; Freeberg
et al., 2012; Maestripieri, 1995; Scopa &
Palagi, 2016). Interindividual tolerance makes
the outcome of most interactions unpredictable
due to the limited influence of rank (despotism)
and kinship (nepotism) in shaping social
relationships (Butovskaya, 2004; Freeberg et
al., 2012). This uncertainty favors a greater
and more sophisticated communicative repertoire
to reduce the risk of misunderstanding (Flack
& De Waal, 2004; Maestripieri, 1995;
Preuschoft et al., 1998; Scopa & Palagi,
2016; Whiten & van Schaik, 2007). The more
evident the signal, the clearer the
message.
-
- The uncovered gum yawn is also elicited by
anxious stimuli (Baker & Aureli, 1997;
Hadidian, 1980; Leone et al., 2014; Liang et
al., 2015; Miller et al., 2012; Moyaho &
Valencia, 2002; Schino et al., 1990; Vick &
Paukner, 2010; Zannella et al., 2015). However,
the extent to which anxiety is experienced by
subjects strictly depends on social organization
and subjects' levels of tolerance. Uncovered gum
yawns and scratching frequencies were higher in
subordinates than in dominants in M. fuscata but
not in M. tonkeana. This result is in agreement
with the resource inequity hypothesis, which
predicts that in despotic societies, dominants
will limit low-ranking subjects' access to
resources by attacking and intimidating them.
The result is that subordinates experience
higher levels of anxiety (Sapolsky, 2005). In
contrast, egalitarian societies have more equal
resource distribution, and anxiety is not
directly linked to low-ranking status (Sapolsky,
2005). However, in tolerant species, at least at
an immediate level, the linkage between yawning
and anxiety is not totally absent. To
investigate whether yawning was a short-term
anxiety-based display, we compared the frequency
of yawning in the postconflict and matchcontrol
conditions. We never recorded the covered teeth
variant after displays of aggression in either
species. As predicted by the uncertainty
relationship hypothesis, uncovered gum yawns, as
for scratching, were significantly higher after
an aggressive event only in Tonkean macaques
compared to the control condition. In this
species, the unpredictability due to the high
risk of renewed aggression is elevated.
Moreover, immediately after agonistic events
involving subjects who share strong bonds, the
anxiety response can be even higher because the
good relationship between aggressor and victim
is at risk (Aureli & Schaffner, 2002;
Kutsukake & Castles, 2001). The agonistic
events are apparently one of the potential
variables affecting uncovered gum yawns and
scratching, which are good indicators of
short-term anxiety, at least in tolerant
species.
-
- Our study highlights the substantial
stability of covered teeth yawns as a function
of (a) social tension conditions (no differences
between the around-food and relaxed conditions),
(b) aggressive contexts (no differences between
the postconflict and matchcontrol observations),
and (c) dominance hierarchy (no correlation as a
function of ranking position; see Figure 4).
Therefore, the covered teeth yawn is a form of
yawning that appears not to be susceptible to
social and environmental stimuli, although
future research should investigate the possible
relation between covered teeth yawns and
endogenous and physiological changes (e.g., the
sleep&endash;wake cycle transition). However,
the uncovered gum yawn is a more evident form of
yawning (canines are completely visible) whose
performance seems to be highly sensitive to all
of the aforementioned variables (see Figure 4).
This plasticity indicates that the uncovered gum
yawn is most likely modulated according to
differences in social contexts.
-
- It is possible that the communicative
function of yawning could be favored by natural
selection, especially in tolerant species, which
need to elaborate more complex forms of social
communication. Research on yawning could be
advanced by further comparative studies in a
wider range of primate societies varying in
social styles, systems, and the degree of canine
dimorphism (see Figure 5). In particular, for
full comprehension of the social functions of
yawning, future studies should focus on its
variability by taking into account not only
frequency but also other factors such as
morphology, duration, and contagious
nature.
-
-
|



