- mise à jour
du
- 8 javier
2003
- Neuropsychopharmacology
- 2004; 29;
158-170
- lexique
|
- Marijuana
withdrawal in humans: effects of oral THC or
divalproex
- Margaret
Haney, Carl L Hart, Suzanne K Vosburg,
Jennifer Nasser, Andrew Bennett, Carlos Zubaran
and Richard W Foltin
- Division on Substance Abuse,
New York State Psychiatric Institute, Department
of Psychiatry, College of Physicians and
Surgeons of Columbia University, New
York
|

|
- ABSTRACT
- Abstinence following daily marijuana use can
produce a withdrawal syndrome characterized by
negative mood (eg irritability, anxiety,
misery), muscle pain, chills, and decreased food
intake.
-
- Two placebo-controlled, within-subject
studies investigated the effects of a
cannabinoid agonist,
delta-9-tetrahydrocannabinol (THC: Study 1), and
a mood stabilizer, divalproex (Study 2), on
symptoms of marijuana withdrawal. Participants
(n=7/study), who were not seeking treatment for
their marijuana use, reported smoking 6-10
marijuana cigarettes/day, 6-7 days/week. Study 1
was a 15-day in-patient, 5-day outpatient,
15-day in-patient design.
-
- During the in-patient phases, participants
took oral THC capsules (0, 10 mg) five
times/day, 1 h prior to smoking marijuana (0.00,
3.04% THC). Active and placebo marijuana were
smoked on in-patient days 1-8, while only
placebo marijuana was smoked on days 9-14, that
is, marijuana abstinence.
-
- Placebo THC was administered each day,
except during one of the abstinence phases (days
9-14), when active THC was given. Mood,
psychomotor task performance, food intake, and
sleep were measured. Oral THC administered
during marijuana abstinence decreased ratings of
'anxious', 'miserable', 'trouble sleeping',
'chills', and marijuana craving, and reversed
large decreases in food intake as compared to
placebo, while producing no intoxication. Study
2 was a 58-day, outpatient/in-patient
design.
-
- Participants were maintained on each
divalproex dose (0, 1500 mg/day) for 29 days
each. Each maintenance condition began with a
14-day outpatient phase for medication induction
or clearance and continued with a 15-day
in-patient phase. Divalproex decreased marijuana
craving during abstinence, yet increased ratings
of 'anxious', 'irritable', 'bad effect', and
'tired.' Divalproex worsened performance on
psychomotor tasks, and increased food intake
regardless of marijuana condition.
-
- Thus, oral THC decreased marijuana craving
and withdrawal symptoms at a dose that was
subjectively indistinguishable from placebo.
Divalproex worsened mood and cognitive
performance during marijuana abstinence.
-
- These data suggest that oral THC, but not
divalproex, may be useful in the treatment of
marijuana dependence.
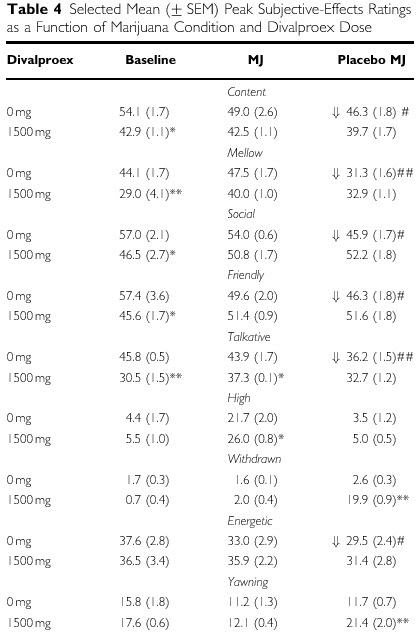
- Subjective-Effects Ratings
- Figure 5 and Table 4 portray the average
daily ratings on selected VAS scales during each
day of each marijuana condition. Data will be
discussed by first describing any significant
effects of marijuana abstinence by comparing
placebo baseline conditions to placebo
abstinence conditions, and then the effect of
divalproex under each marijuana condition will
be described.
-
- Table 4 shows that under placebo divalproex
maintenance, ratings of 'Content', 'Mellow',
'Social', 'Energetic', 'Friendly', and
'Talkative' were all decreased during marijuana
abstinence compared to baseline. In terms of the
effects of maintenance condition during each
marijuana condition, divalproex under baseline
conditions decreased ratings of 'Content',
'Mellow', 'Social', 'Friendly', and 'Talkative'.
During active marijuana smoking, divalproex
continued to decrease ratings of 'Talkative',
while significantly increasing ratings of 'High'
compared to placebo. Figure 5 demonstrates that
divalproex substantially worsened ratings of
'Anxious', 'Irritable', 'On Edge', and 'Sleepy'
during marijuana abstinence.
Divalproex also increased
ratings of 'Withdrawn' and 'Yawning' during
marijuana abstinence (Table 4).
|


